February 7, 2014- By Steven E. Greer, MD
The Patient Centered Outcome Research Institute (PCORI) was created by the PPACA “ObamaCare” law in 2010. Well-funded with approximately $3 Billion over ten years, the mission was, among other things, supposed to be to conduct comparative effectiveness research (CER) that would determine whether costly therapies are any better than cheaper alternatives.
The rise of CER has been one of the most feared developments by the drug and device industries. To avoid powerful lobbying efforts that could have resulted in de-funding and the death of PCORI before it got started, the institute steered away from even hinting at conducting CER. Now, almost four years later, PCORI is finally funding CER research. However, critics, such as former White House Director of Office of Management and Budget, Peter Orzag, say that the money spent by PCORI on CER is still not enough.
Meanwhile, a leading doctor in charge of PCORI research strategies, Harlan Krumholz, MD, Read more »
March 15, 2014- By Steven E. Greer, MD
In 2009, Atul Gawande, MD, MPH and his large international team published in the New England Journal of Medicine (NEJM) an observational study that showed a significant reduction of death and “complications” after non-cardiac surgery. The World Health Organization (WHO) created the checklist used in the NEJM paper. After this non-randomized, non-controlled, observational study was published, entire nations adopted the surgical checklist system.
Now, in 2014, a population study drawing from Ontario surgical patient data, published in the NEJM, showed no significant benefit from the widespread adoption of the same WHO surgical safety checklist that Dr. Gawande popularized. This study was also observational, but it was stronger than the 2009 Gawande study in that it included the entire population within a region.
What went wrong? Read more »
 Anesthesiology, Biostatistics, Cardiac surgery, Critical care, General Surgery, Harvard affiliates, HHS, Infectious Disease, NEJM, NICE (UK), Op-Ed, Orthopedic surgery/sports, Plastic Surgery, Policy, Preventive Medicine, Spine surgery, Trauma, Vascular Surgery | apples49 |
Anesthesiology, Biostatistics, Cardiac surgery, Critical care, General Surgery, Harvard affiliates, HHS, Infectious Disease, NEJM, NICE (UK), Op-Ed, Orthopedic surgery/sports, Plastic Surgery, Policy, Preventive Medicine, Spine surgery, Trauma, Vascular Surgery | apples49 |  November 8, 2014 10:24 pm |
November 8, 2014 10:24 pm |  Comments (0)
Comments (0)
Interviewed and produced by Steven Greer, MD
Erick Turner, MD, psychiatrist at Oregon Health and Science University and former FDA medical officer reviewing psychotropic drugs, discusses his new England Journal of Medicine paper on the problem of selectively publishing only the positive trials on antidepressants and the failure of drug companies to publish the negative trials, thus skewing the body of literature.
March 20, 2014-By Steven E. Greer, MD
Charlie Rose interviewed Google founder Larry Page at the 2014 TED conference. During the talk, Mr. Page unabashedly announces that he thinks your most sensitive of information, your medical records, should be made public for “research”. He naively believes that these records can be “anonymous”.
Google wants your medical records
Google wants your DNA now (Not just with 23andMe)
Beware The Google
The ARBITER 6-HALTS study investigating Zetia and Niaspan was stopped early, confounding the outcome and likely inflating the efficacy of Niaspan. There was no ethical reason to stop the trial early. It is quite possible that there would have been no benefit to Niaspan whatsoever if the trial were carried out to completion.
Dr. Gordon Guyatt discusses the statistical reasons that clinical trials should not be stopped early, in most cases. Early stoppage has been a growing problem in the cardiology and oncology trials.
Gordon Guyatt, MD, epidemiologist, internist, and biostatistician at McMaster University updates us on the progress being made against the problem of clinical trials being stopped early to inflate efficacy of drugs. Since our first coverage of the topic two years ago, the FDA, the Cochrane group, and other agencies have implemented changes in policy. Dr. Guyatt discusses the Crestor JUPITER trial as an example of a trial that was stopped early and inflated efficacy.
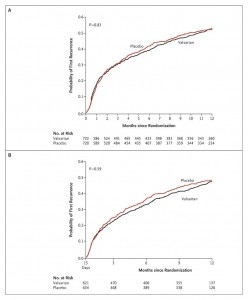
Dr. Guyatt explained in a previous post why early stoppage of clinical trials results in falsely inflated efficacy of the therapy being studied. The April 16, 2009 edition of The New England Journal of Medicine has a great example how Kaplan curves tend to converge with time and erase early efficacy differences. The study looked at the drug valsartan for the treatment of atrial fibrillation and failed to show a benefit over the comparator cohort(see graph). If it were stopped early, it would have shown that the drug was effective. Note the curves touching, or converging, as time progresses.
Archie Bleyer, MD, radiation oncologist at the Oregon Health and Science University, discusses his NEJM paper that concluded early breast cancer screening using mammography led to more findings of false positives.
In full screen 1080i HD for better viewing of the data graphics
and NBC’s egregious coverage



